SRS image stack series workflow
Purpose
Use the SRS image-stack series workflow to collect image stacks using systematic random sampling (SRS). You can then use these image stacks with unbiased stereology probes such as Optical Fractionator, rather than conducting a stereological study on live-camera images (see Counting offline after an SRS Image Stack acquisition).
This is useful when:
- You want to minimize fluorescence-signal bleaching.
- You have tissue labeled with more than a single fluorophore that you want to compare.
- You want to archive the image stacks.
Before you start
Microscope systems are subject to a phenomenon called thermal drift: once power is on, the hardware begins to heat up, which can cause the focal plane to shift by several micrometers. To avoid thermal drift, we recommend that you power on the microscope and stage at least 2 hours before acquisition.
Accurate tissue thickness measurements are critical for stereology. When working with image stacks, the accuracy of your thickness measurements is limited by the distance between planes in your image stack. A popular workaround is to measure your tissue’s thickness in live image mode, as follows
Measure the tissue’s thickness separately from counting:
- Use the DAPI channel (if present), and go to approximately 15 random sites across all your sections.
- At each site, focus on the top and bottom, note the values and subtract these numbers to get the thickness.
- Average the thickness for all sites.
- Proceed to counting.
- Once you're done counting, go to Probes>Probe Run List and click the View results button.
- Click the Edit Section cut Thickness button and enter the average thickness.
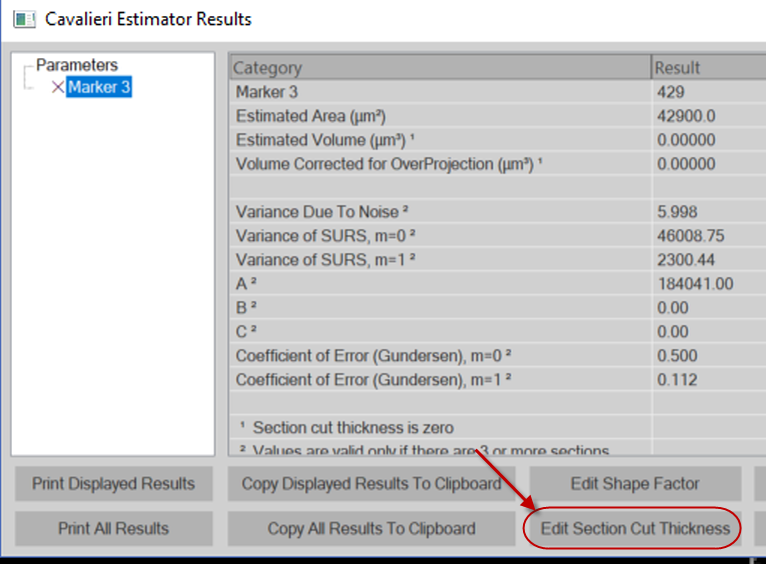
- Use the Estimated Population Using User Defined Section Thickness provided in the Probe Results window.
Only use this estimate for this purpose. If you are working in live image mode, use one of the other 3 population estimates instead.
- Click the Edit Section cut Thickness button and enter the average thickness.
It's a good idea to conduct one or more practice runs on material that is similar to, but less precious than your experimental material. Use these practice runs to identify the ideal image-acquisition settings. Following are some suggestions for becoming familiar with SRS acquisitions and settings.
- Watch this video tutorial on fluorescence imaging. Acquire a few practice image-stacks to make sure that you understand the settings and that there is no bleaching before you get too far into your project.
- Increase the acquisition speed: Go to Acquire>Multichannel Control; click the Setup button then check the Acquire the channels [...] button.

- Keep your exposure times low (less than 200 ms) if possible.
-
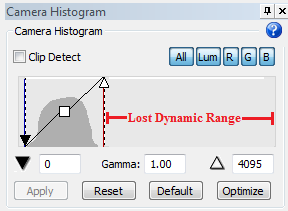
When adjusting your multichannel settings, keep in mind that there will be areas that are much brighter or darker and that you can’t change these settings once you've started the acquisition. We recommend that you adjust the camera histogram as follows: Drag the white point to the left (and leave some space to the right of the histogram) to shorten your dynamic range, like this:
Why this is important
There will be areas of your tissue that are very bright, and others that are very dim. Be aware that if you optimize your camera settings for the dim areas, the bright areas will be completely saturated and unusable; on the other hand, if you optimize your camera settings for the brighter areas, the dim areas will still be usable, albeit grainy (this is what we recommend). The problem is that, when you are setting up your camera settings, you can't know whether you are looking at a bright area, a dim area, or something in the middle. We recommend that you treat the area as a bright area, and set up your histogram with a shorter dynamic range as shown above.
- Once you've finished acquiring, you can also make adjustments: Use Image>Adjust, select the channel you want to change, and click Optimize. Note that it is possible to count from a grainy image (that is, an image with a (low dynamic range) but it is virtually impossible to count cells that are saturated.
- Apart from exposure time, the biggest factor for determining the duration of the SRS acquisition is the distance between images (defined in step 8 of the workflow): The greater the distance, the faster the acquisition. Make sure that you allow at least three planes per cell. For example, if the cell you want to count is 20 μm across, there is a 50% tissue shrinkage, and you want 3 planes per cell; then set your distance between images to 3 μm.
- You can trace more than one section at a time. Most slide holders can hold at least two slides, so it's possible to trace several sections at once, put a drop of oil on each and let them run over the weekend. Because you are limited to a single parameter set per run of the workflow, multiple ROIs may not be practical if you need a different grid size for them. In this case, acquire one ROI first, then run the workflow again to acquire the next one, and so on.
Procedure
- Set up the slide at the desired magnification on a calibrated system.
- Click Acquire>Display>Live Image to view the tissue on the screen.
- Adjust settings to optimize the display of the tissue (i.e., adequate white balance, light, background, etc.).
- Click Acquire>Workflows>SRS image stack series.
-
Follow the workflow steps.
Workflow overview
Areas to Acquire
Acquire Configuration
Acquire
After the SRS acquisition
Before opening your data file:
- Go to File>Preferences and select the Images panel.
- Turn off (uncheck) Zoom to fit on load.
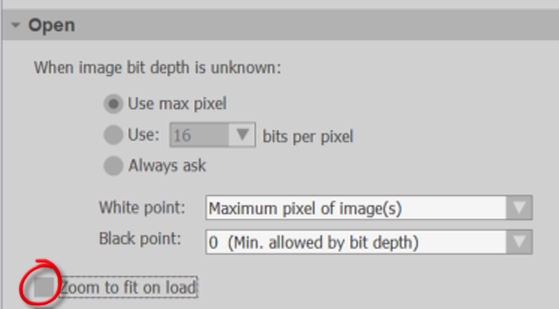
- Turn on (check) Remove off-screen image stacks from memory.

Many users combine SRS image stacks with the Optical Fractionator to count cells (see Using Optical Fractionator to count offline after an SRS Image Stack acquisition ).
When counting:
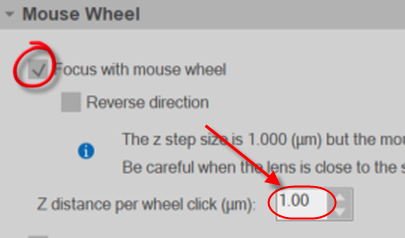
- Go to File>Preferences and select the Movement panel.
- Turn on (check) focus with mouse wheel and set the Z distance per wheel click equal to the distance between images in your image stack. This will enable you to focus with the mouse wheel instead of the page up/page down keys.
Try to avoid using the Image Adjustment window – making changes to each stack is time consuming. If you need to use it, remember to save every few sites (otherwise, the software will keep unsaved images open in memory, slowing the system down if you have many sites).
If you are struggling with the automatic zoom feature (that is, you have to zoom out at each site to see the counting frame), contact us.