Spaceballs workflow
Purpose
|
|
The Spaceballs probe is used to perform a systematic random sampling of a predefined 3D region of interest and estimating the total length of many different types of objects or fibers, including tubules, nerve fibers, small blood vessels, surfaces, and microvilli. Spaceballs provides a length estimate instead of length-density. Reporting length per region instead of length per volume is more effective because a length-density measure can't account for possible concomitant changes in volume along with length. The Spaceballs workflow steps you through the process of setting up to use the probe and mark/count your objects of interest. |
How it works
The probe is a sphere or hemisphere virtually embedded in the tissue, and the counted parameter is the number of profiles that transect the edge of the sphere within a defined counting region. Since the surface of a sphere is isotropic, the need for isotropic uniform random sections is eliminated.
Spaceballs is implemented with a fractionator sampling methodology to return an estimate of total length per region. A series of sites are selected within each section by systematic random sampling. At each site, a 3D sphere of constant volume is superimposed upon the slide to represent the intersection sites to be counted. The item to be counted is a "profile," that is, the point where the fiber transects the sphere boundary. If fibers are thick, you must identify the center point of the fiber, and only count locations where the center point transects the sphere outline.
Edge cases
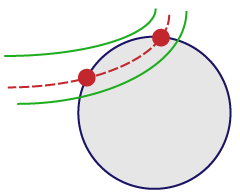
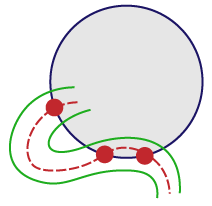
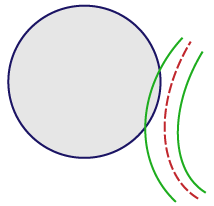
For vessels that appear on the edge of the spaceball, use the “center line” rule:
|
|
|
|
If the center line enters and then leaves the probe, mark it twice (at the entrance point and at the exit point). |
If the center line weaves in and out, mark the center line every time it enters or leaves the spaceball. |
If the center line doesn't enter the spaceball, don’t mark it. |
Requirements
- Thick sections (significantly thicker than the diameter of the objects to be measured).
- Structure of interest that is stained and visible through the depth of the tissue section.
- Thin focal planes achieved with a high magnification, high numerical aperture lens, generally oil immersion.
- Approximate section thickness: Use a high power objective lens with a high numerical aperture to focus at the top and bottom of a few sites throughout your sections to get a rough idea of the section thickness. This value can be edited later, but an approximate value is needed for setting up the disector and guard zones.
We recommended that you place a reference point in an easy to find and recognizable location before starting this probe workflow. When working with a live camera feed, the placement of the reference point is essential for maintaining accurate alignment between a data file and its corresponding experimental sample when collecting data over multiple sessions. If a reference point has not been placed before opening a workflow, Stereo Investigator will automatically place it in the top left corner of the main window. This location may not be ideal for resuming data collection in subsequent sessions.
Starting the Spaceballs workflow
-
 Click the Spaceballs workflow button on the Probes ribbon.
Click the Spaceballs workflow button on the Probes ribbon. If you haven't used the workflow recently, find the Spaceballs workflow button in the length drop-down menu in the All Probes section of the Probes ribbon.
If you haven't used the workflow recently, find the Spaceballs workflow button in the length drop-down menu in the All Probes section of the Probes ribbon. -
In the dialog box that opens, the following choices may be available, depending on what files, if any are currently open. Choose what you would like to do:
- Start a new subject: Count objects on a live-camera feed from your microscope or in an image file, containing the entire region to be counted that you have already opened.
- Continue working with this subject: Continue working in an open data file.
- Load subject data from existing file: Continue working in a stored data file that is not currently open. Choosing this option may jump you directly to the Count objects step.
- Start a new subject from image stacks: Load image stacks acquired in a systematic random sampling (SRS) manner acquired using another microscope system (i.e., not an MBF Bioscience system).
Spaceballs workflow steps
The workflow is dynamic; your choices determine which steps are included, and what information is requested and displayed. Because of this, the step numbers you see onscreen in Stereo Investigator may be different than those in the complete workflow described here.
Click any of the underlined steps to jump to the instructions:
Indicate the Areas Used for Counting
Define Probe Configuration
Perform Counting
Commands available in every step of the workflow:

|
New workflow: Click the new workflow button to start over; the settings will revert to the defaults or those specified in the previous completed workflow. |

|
Previous step / Next Step: Click to advance in the workflow or revisit a previous step. Alternatively, you can click the steps listed at the top of the workflow to jump to that step. |
|
|
Click the help link to view information in the User Guide on completing the current step in the workflow. |
