Slide scanning: Troubleshooting for fluorescence
Uneven illumination / vignetting / tiling / lines
There are multiple possible causes. We recommend that you start by acquiring a 3x3 test image with all your channels, keeping the exposure times low (less than 200 ms to limit bleaching) and examine the results.
The issue is likely related to the light source. Here are some options to resolve this issue:
- Make sure that your light source is centered and aligned. Because the procedure for this varies from light source to light source, we recommend that you contact your vendor, local microscope representative, or someone from the MBF Bioscience Tech Services team.
Many direct-coupled light sources (incl. Zeiss Colibri, X-Cite 120LED, X-Cite 120 Boost) do not have centering options.
Liquid light guide light sources (e.g., Lumencor SOLA) can potentially have problems with the liquid light guide cable. Most liquid light guide cable manufacturers recommend replacement every two years, regardless of usage. Replacements can be purchased through the manufacturer or MBF Bioscience.
OR
- Increase the trim/blend settings in Step 7 of the workflow. Note that this may hide rather than fix the problem, will increase acquisition time, and may worsen bleaching of adjacent sites (see below). Typically a trim of 20 - 100 pixels is recommended.
OR
- Use background correction (Step 6 of the workflow). This may alter the signal-to-noise ratio in your image.
When scanning fluorescent tissue, the light source tends to cover a larger area of tissue than the camera’s field of view. 
This is problematic when scanning the next sites because the area that was hit by light previously gets bleached, and it results in “vignetting” or “lines” in the scan. 
In addition, the camera histogram is typically optimized to obtain a wide dynamic range. That is, if your histogram looks like this: 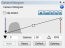 , you would typically increase the exposure time so that the histogram looks more like this:
, you would typically increase the exposure time so that the histogram looks more like this: 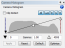 . The problem is that increasing the exposure time with tissue that is prone to bleaching exacerbates the “adjacent tile” bleaching issue (often substantially).
. The problem is that increasing the exposure time with tissue that is prone to bleaching exacerbates the “adjacent tile” bleaching issue (often substantially).
This type of problem is less common with tissue that’s been properly stained and mounted so it may be worth investigating your tissue preparation protocol for any opportunity to improve the stability of your fluorophores.
If this issue occurs in the DAPI channel (usually the most robust channel), that clearly points towards a problem during tissue preparation. It is less clear for other channels; there are certainly some fluorophores that are much more prone to bleaching than others.
With cameras that have a dynamic range greater than 8-bit (e.g., Hamamatsu cameras), you can lower the dynamic range by moving the white point slider, like this: 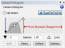 This approach will allow you to use a lower exposure time, and the decreased dwell time on each site will prevent a lot of the bleaching.
This approach will allow you to use a lower exposure time, and the decreased dwell time on each site will prevent a lot of the bleaching.
This is not recommended with an 8-bit camera as it will result in a grainy image.
Use our illumination correction tool (in the Image ribbon bar) to correct this issue. It is likely to work well but it greatly increases the time required for that acquisition (more than double) when applied during the acquisition, and it can’t be applied to images opened in virtual image mode (that is, to slide scans) after acquisition.

Zeiss makes two apertures: a square one (the XY) for the F slot, and a circular one for the A slot.
- If the F slot is not in use, insert the XY aperture in the F slot.
- If you have an ApoTome in the F slot, do not remove it; use the A slot instead to insert the circular A aperture.
- Only use the A slot for the aperture if you are equipped with an ApoTome.
- The A aperture may partially help with this issue, but it will not be as effective as the XY aperture.
- Closing down either of these apertures too much will exacerbate the uneven illumination.
Both apertures are shown in the photo for illustration only; they should NEVER be used together!
It may help although LEDs typically run at 100% intensity to keep exposure times as low as possible.
Decreasing the intensity will probably require a higher exposure time, which may result in a similar amount of bleaching, and will cause slower scans – meaning that there’s basically no advantage to lower light intensity in most cases.
Background correction will not help with this, since it is caused by bleaching of adjacent sites, and not a truly uneven illumination.
To rule out bleaching as the cause for uneven illumination, we recommend acquiring an image with a Chroma background correction slide.
There are Chroma blank slides for each channel; these slides provide a uniform illumination and their intensity is even throughout the field of view.
You can purchase those slides through Chroma or MBF Bioscience.
This may be the result of a filter cube issue.
To resolve this, move the filter cube to a different position, make sure it's properly seated and secure, and then replace the cube.
Note that:
- With high exposure times (above 300-500 ms), a variety of artifacts can appear, especially random red/green/blue pixels on color cameras.
- Older cubes do burn out, but we generally only recommend replacing a cube if, for example, there is no trace of uneven illumination for red and green but blue shows uneven illumination, even with an appropriate exposure time and a known appropriate light source.
- Some light sources (such as the X-Cite 120 LED) use different bulbs for UV (DAPI) and other channels, so it's possible (albeit rare) to have a light source issue that occurs only in one channel.
Try using the focus map's heat map (Step 9 of the workflow) to look for large changes in Z before the acquire starts. Large Z differences between sites can cause out of focus sites that look like uneven illumination.
It is common to have some areas of the tissue that are brighter or darker than others. Make sure that you optimize your camera histogram for the brightest area you are interested in, and adjust it post-acquisition using the Image Adjustment panel to improve dimmer areas' appearance (gamma adjustment is typically a good option).
Increase the number of focus sites ONLY if certain areas are darker because they are out of focus, not simply because they’re dark.
Other issues
To solve this issue, review your calibration.
To solve this issue, adjust the histogram (this can be done before the acquisition [see Camera Histogram guide], or after the acquisition [see Image Adjustment guide]). For best practices, refer to this tutorial video: Fluorescence imaging.
The is most likely the result of thermal drift. To avoid this issue, power your hardware on at least 2 hours before you set up your focus map and make sure that there is no hot or cold air blowing on it.
You may also need to add sites to your focus map. See step 9 of the slide scanning workflow.