Scientists use Vesselucida 360 to quantify brain vasculature in mTBI model
It is not uncommon for war veterans returning home from war-zones like Iraq and Afghanistan to suffer from blast-induced traumatic brain injuries (TBI). In these situations, the most common types of blasts are lower level blasts, the kind that produce mild TBIs (mTBI). Though the effects of a mTBI aren’t visible from the outside, scientists say the blood vessels inside the brain are deeply altered.
In their study of a mouse model of mTBI that mimics the blast exposure associated with human mild TBI, a research team, that includes MBF Bioscience Scientific Director Dr. Susan Tappan, say that low-level blast exposure disrupts the way cells interact with each other within the brain’s neurovascular unit.
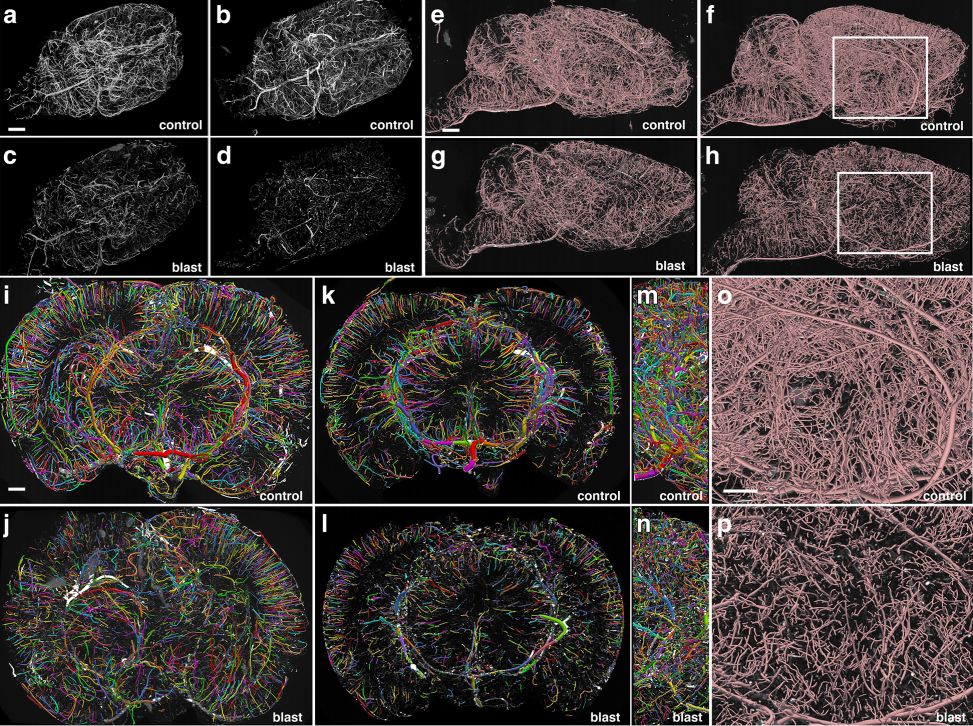
Fig:1 Chronic vascular pathology in blast-exposed rats revealed by micro-CT scanning. Two control and two blast-exposed rats were transcardially perfused with the Brite Vu contrast agent at 10 months after blast exposure. Brains were scanned at a resolution of 7.5 μm using equispaced angles of view around 360°, and 3D reconstructions were prepared with Bruker’s CTVox 3D visualization software. a-d MIP images of volume-rendered brain vasculature from two control (a, b) and two blast-exposed (c, d) rats revealed diffuse thinning of the brain vasculature in the blast-exposed rats. Scale bar, 2 mm. e-h Trace sagittal reconstructions used for the automated quantitation from control (e-f) and blastexposed rats (g-h) o-p Higher magnification views of the regions outlined by the boxes in panels (f) and (h). Scale bars, 1 mm for (e-h), and 0.6mm for (o-p). i-n Reconstructions of coronal optical sections from the brains of control (i, k) and blast-exposed (j, l) animals. Panels (i) and (j) correspond approximately to coordinates interaural 12.24–9.48 mm and panels (k) and (l) correspond approximately to coordinates interaural 6.94–3.24 mm. Lateral views of (i) and (j) are shown in (m) and (n), respectively. Vessels were color coded to allow visualization of individual vessels automatically traced by the Vesselucida 360 software. Note the general loss of radial organization in the blast-exposed shown in panel (j). Scale bar, 1 mm for (i-n)
Aiming to mimic an event often experienced by soldiers and military personnel in war-torn regions, the scientists exposed rats to a series of three blasts — one blast per day, over three consecutive days. Though the rats developed behaviors typical to chronic PTSD, their neuronal pathology, at least at the light and electron microscopy levels remained unchanged, according to the study. However, when the researchers examined the rat brains on a vascular level, they found evidence of chronic damage.
One molecular change they observed was a decrease in vascular-associated glial fibrillary acidic protein (GFAP) levels in brain vascular fractions six-weeks after blast exposure. These low levels of GFAP tipped the researchers off to the possibility that gliovascular and neurovascular interactions were being disrupted in mTBI brains.
Another clue that neurovascular connections were compromised was the disappearance of a series of several neuronal intermediate filament (IF) proteins. The disappearance of these proteins, which are important signals of healthy brain function, led the researchers to delve deeper into the brain’s vascular unit.
To quantify the rats’ cerebral vasculature, the research team used Vesselucida 360 to analyze micro-CT images of the rat brains 10 months after blast exposure. Using data from the micro-CT scans, Vesselucida 360 automatically reconstructed the vascular networks in 3D, color coding individual vessels for more efficient visualization. With these reconstructions, the researchers observed a decrease in total brain vascular length (about 50 percent), a decrease in total surface area (over 50 percent), and a decrease in total volume (about 60 percent) in rats who suffered from blast-induced mTBI as compared to the control group.
Their reconstructions also revealed that mTBI brains showed a major disruption of the neatly organized, radial patterns typical of blood vessels in a healthy brain.
Interestingly, GFAP and IF protein levels returned to normal after eight months, but despite this recovery, chronic vascular abnormalities remained — an observation consistent with prior studies.
“These findings suggest that normalization of GFAP and IF protein levels in isolated vascular fractions, while likely signaling the tightening of gliovascular and neurovascular connections, does not translate into a resolution of the vascular pathology.”
Gama Sosa, M.A., De Gasperi, R., Perez Garcia, G.S., Perez, G.M., Searcy, C., Vargas, D., Spencer, A., Janssen, P.L., Tschiffely, A.E., McCarron, R.M., Ache, B., Manoharan, R., Janssen, W.G., Tappan, S.J., Hanson, R.W., Gandy, S., Hof, P.R., Ahlers, S.T., and Elder G.A., Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain, Acta Neuropathologica Communications (2019) doi.org/10.1186/s40478-018-0647-5
https://actaneurocomms.biomedcentral.com/articles/10.1186/s40478-018-0647-5


