Scientists Use Neurolucida in Study Describing New Cell Population in Juvenile CA3 Hippocampus
There’s a lot to be said for being in the right place at the right time. For a neuron, emerging at a certain place within the brain destines it for a particular function. A new study posits that, for a group of cells in the hippocampus, it’s not only where a neuron is born, but also when it is born, that defines the specific roles it will play. The study, conducted by researchers at the Mediterranean Institute of Neurobiology (INMED, France) and affiliated institutions, identifies a new population of cells in the hippocampus.
The cells identified are “a sub-population of early-generated glutamatergic neurons that impacts network dynamics when stimulated in the juvenile hippocampus,” according to the paper.
“These cells first operate as assemblies, in the developing hippocampus, and later become powerful single units capable of triggering network synchronization in the absence of fast GABAergic transmission,” Marissal et al. say.
In the paper, the authors cite prior research that describes how neurons in the CA3 hippocampus called hub neurons achieve network synchronization, generating giant depolarizing potentials (GDPs). But the neurons identified at the time were all GABAergic. The glutamatergic cells, which comprise the majority of cells in the network, couldn’t generate GDPs when stimulated.
Based on these earlier findings, Marissal and his colleagues speculated that glutamatergic cells did not play that big of a part in the physiological synchronization processes of neurons in early postnatal periods. But what would happen if GABAergic activity was blocked in a developing hippocampus?
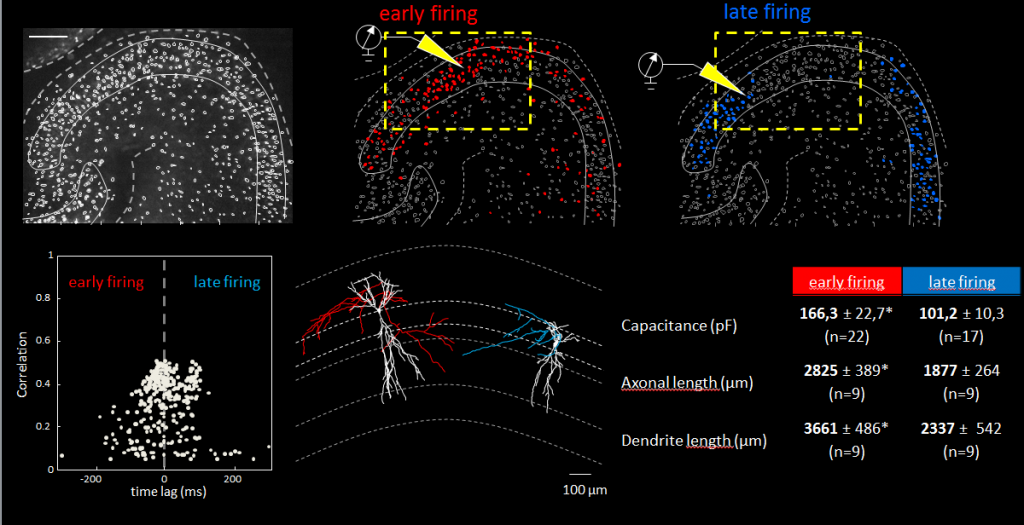
Using an array of in vitro techniques (including fate-mapping, calcium imaging, and patch-clamp recording) and neuron reconstruction, the scientists analyzed neuronal activity in the developing brains of young mice after blocking GABAergic transmission. They also examined the involvement of early generated glutamatergic neurons (EGNs) and late generated glutamatergic neurons (LGNs) in the network.
They found that network bursts could be triggered by new EGNs collaboratively, or by more mature EGNs acting individually. They also determined that neurons were recruited into “network bursts” according to the timing of their birth with EGNs being recruited before LGNs.
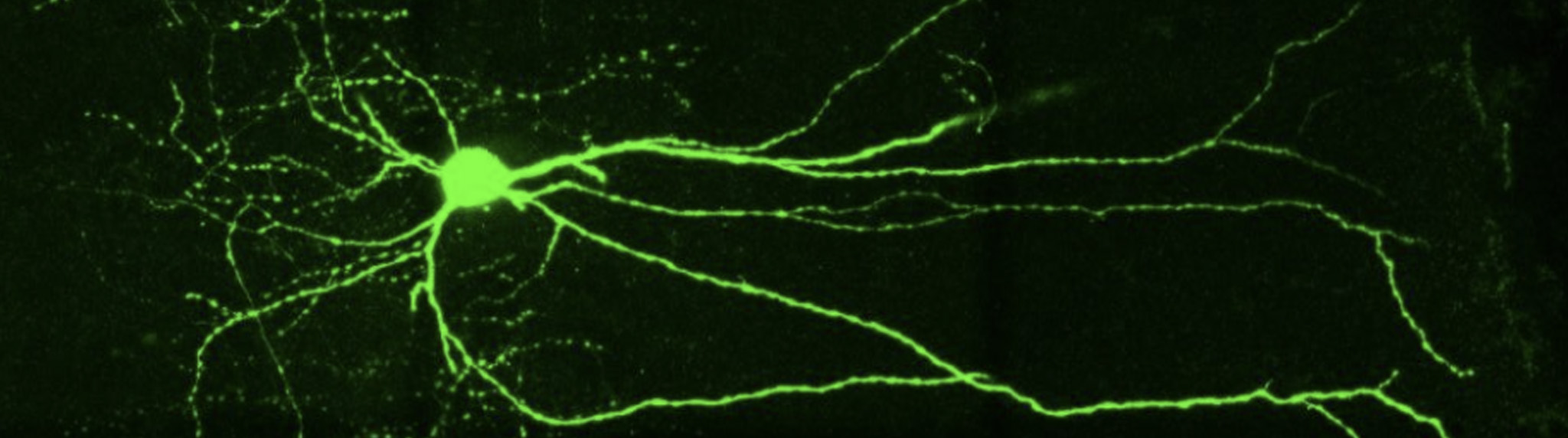
“In this study, Neurolucida helped us describe a new subfamily of CA3 pyramidal neurons that originates from the earliest stages of embryogenesis. [Through neuron reconstruction] we were able to show that they have long primary apical dendrites and an axon with many ramifications.” said Dr. Rosa Cossart, a co-author of the study who has been using Neurolucida in her research for over a decade.
“The fact that ‘early-born’ cells are central nodes in the functional organization of hippocampal networks may well be a general principle albeit with important differences between GABAergic and glutamatergic neurons, the former but not the latter exerting their roles at an early developmental stage,” the authors say. “We propose that the temporal origin of neurons is a critical determinant of single-cell function.”
Read the full paper at Nature Communications.
Marissal, T., Bonifazi, P., Picardo, M. A., Nardou, R., Petit, L. F., Baude, A., … & Cossart, R. (2012). Pioneer glutamatergic cells develop into a morpho-functionally distinct population in the juvenile CA3 hippocampus. Nature communications, 3, 1316. doi: 10.1038/ncomms2318